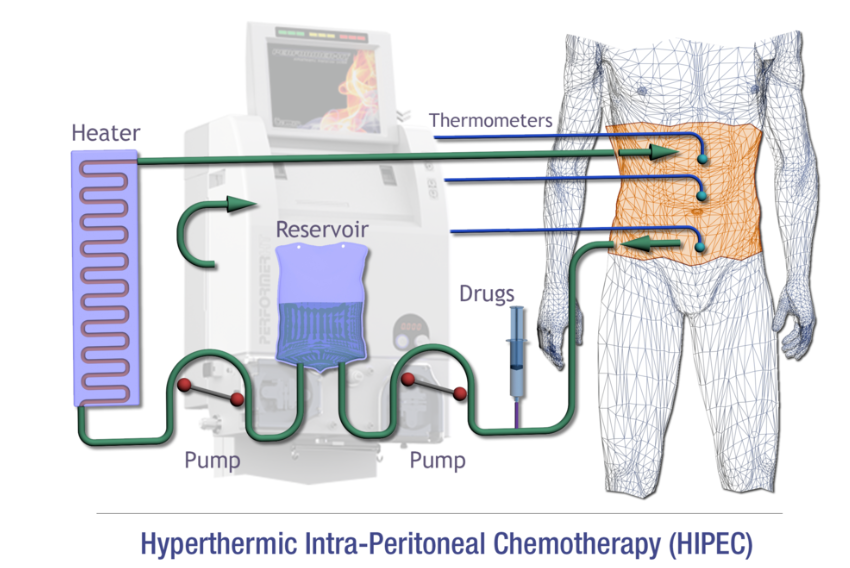
A medical stay in Turkey offers patients access to advanced treatments such as hyperthermic intraperitoneal chemotherapy (HIPEC), a protocol in which chemotherapeutic agents are directly administered into the abdomen at high temperatures. This approach is commonly recommended after cytoreductive surgery for ovarian cancer.
The rationale behind HIPEC is that, in advanced stages of the disease with peritoneal dissemination, it is challenging to completely eradicate the cancer (referred to as achieving “residual tumor 0”). In these cases, adjuvant chemotherapy, typically involving platinum derivatives combined with Taxol, is often proposed. However, even with successful treatment, patients face a high relapse rate—around 70% within five years. To address this, adding intraperitoneal chemotherapy during a medical stay in Turkey aims to improve survival rates and reduce the chances of recurrence, providing patients with a cutting-edge treatment option in the country.
A little history
The first experimental clinical study dates back to 2006: the GOG (Gynecological Oncology Group) 172. This study included 415 patients with stage III ovarian cancer who had undergone cytoreductive surgery, with a residual tumour of around 1 cm. Despite the presence of complications and low patient compliance, treatment with HIPEC demonstrated positive results, with a mean survival of 65 months versus 49 months in the control group.
However, other clinical studies carried out subsequently, involving both patients with first-line or relapsed ovarian cancer and other tumor types, showed sometimes contradictory results, with no apparent increase in survival linked to the use of HIPEC.
In April 2018, the European Society of Medical Oncology and the European Society of Gynecological Oncology (ESMO-ESGO) decreed that HIPEC should not be considered a standard therapy for the treatment of ovarian cancer.
Treatment rationale: when it’s used
HIPEC is currently used to treat advanced ovarian cancer, but also other types of cancer, such as uterine sarcoma, colorectal cancer, gastric cancer, masothelioma and peritoneal pseudomyxoma. Intravenous chemotherapy is used either directly after reduction surgery, before conventional intravenous chemotherapy, or after surgery and intravenous chemotherapy. The chemotherapies used vary considerably according to the type of disease and the patient’s clinical history, including platinum derivatives, Taxol, Doxorubicin and Melphalan. Chemotherapy is administered directly into the patient’s abdominal cavity, with the temperature maintained at around 37°-43°. Administration takes place in the operating theatre under close medical supervision, and the number of cycles and the distance between one cycle and the next depend on the drug used.
This strategy has two positive implications: on the one hand, intraperitoneal administration is more effective than intravenous administration, as it comes into direct contact with the peritoneal lesions; on the other hand, high temperature itself has an antiproliferative effect and can enhance the effect of antitumor drugs. This effect has been verified down to a depth of 3 to 5 mm, so it makes sense to offer this treatment after cytoreductive surgery.
However, it should be noted that there is no standardized protocol, so parameters such as temperature, speed and method of infusion, interval since surgery, etc. vary from center to center.
What is intrathoracic hyperthermic chemotherapy surgery?
The hyperthermic intraperitoneal chemotherapy procedure in Turkey has proven to be both safe and effective. High-temperature chemotherapy effectively targets tumor cells by penetrating local tissues with minimal circulation, thereby reducing side effects. All organs affected by the tumor are immersed in chemotherapy to eliminate any residual cancer cells.
During the first phase, the HITHOC device fills the chest with a saline solution, gradually heating it to approximately 41°C, a temperature that weakens tumor cells without harming healthy tissue. The device then infuses a higher-than-normal dose of chemotherapy (cisplatin, and possibly doxorubicin) for 45 to 90 minutes. This approach ensures limited diffusion into the bloodstream, allowing the chemotherapy to penetrate just a few millimeters into the thoracic cavity tissues. Finally, the surgeons drain the fluids from the cavity, completing the procedure.